METACYL
Catalytic META C-H ACYLation of arenes
Scheda
Aree / Gruppi di ricerca
Partecipanti al progetto
- Della Ca' Prof. Nicola
- Vinayak Botla (PostDoc)
Descrizione del progetto
The ability to transform inert C–H bonds into reactive functional groups has come to the forefront of modern synthetic developments in recent years. This approach can provide simplification of synthetic routes, new disconnections, and new synthetic starting points by avoiding pre-installed reactive handles. In this direction, the site-selective CH activation and functionalization of aromatic arenes constitutes a challenge of paramount importance in organic synthesis. The use of suitable directing groups together with transition metal-catalysts has enabled the activation of ortho C–H bonds of aromatic rings in multiple versions (Figure 1).
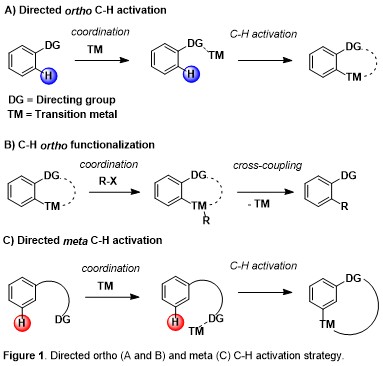 Moreover, if ortho- and para-substitution of aromatic arenes have been quite thoroughly explored by the aid of classical organic synthetic reactions, activation of the meta-position in an arene system remain challenging and, at the same time, essential since this allows the direct green synthesis of several natural products, medicinal drugs and agrochemicals. In particular, C-H acylation reactions represent an outstanding step- and atom-economical tool for the environmentally friendly construction of synthetically useful molecular scaffolds. In striking contrast to ortho C-H acylation of arenes and heteroarenes (directing-group strategy) and para C-H ones (Friedel-Crafts chemistry), meta C-H acylations are almost completely unexplored (Figure 2).
Moreover, if ortho- and para-substitution of aromatic arenes have been quite thoroughly explored by the aid of classical organic synthetic reactions, activation of the meta-position in an arene system remain challenging and, at the same time, essential since this allows the direct green synthesis of several natural products, medicinal drugs and agrochemicals. In particular, C-H acylation reactions represent an outstanding step- and atom-economical tool for the environmentally friendly construction of synthetically useful molecular scaffolds. In striking contrast to ortho C-H acylation of arenes and heteroarenes (directing-group strategy) and para C-H ones (Friedel-Crafts chemistry), meta C-H acylations are almost completely unexplored (Figure 2).
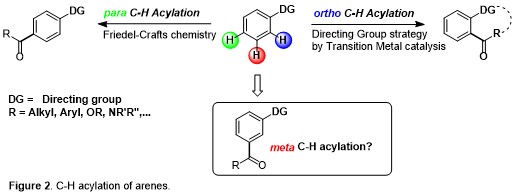
Carbonylative C-H activations can lead to an unprecedented degree of molecular sophistication by combining the atom economical insertion of CO with the green activation of unreactive C-H bonds. In striking contrast to ortho C-H acylation of arenes and heteroarenes (directing-group strategy) and para C-H ones (Friedel-Crafts chemistry), meta C-H acylations are almost completely unexplored. This proposal aims at the development of the first example of meta C-H acylation via mild carbonylation of arenes. Pivoting on this breakthrough, it will be possible to accomplish synthetic methods 1) for the efficient synthesis of hardly accessible organic compounds, 2) for the activation and carbonylation of meta C(sp2)-H bonds, 3) by employing transient directing group and 4) demonstrating the application of these protocols under continuous flow conditions.
Communication of the project on the University website:
https://scvsa.unipr.it/it/notizie/avviato-nellateneo-il-progetto-metacyl-su-nuove-metodologie-la-funzionalizzazione-di
Risultati e pubblicazioni
Pd-Catalysed oxidative carbonylation of α-amino amides to hydantoins under mild conditions.
https://air.unipr.it/handle/11381/2912143
Advances in visible-light-mediated carbonylative reactions via carbon monoxide (CO) incorporation.
https://air.unipr.it/handle/11381/2897963
Note
